In this Chapter
- Getting Started
- What Do I Need To Do To Get Started?
- Figure 3: Office-Based Notification
- The Communication Highway
- Table 2: Drug–Drug Interactions
Getting Started
If Suboxone becomes routinely available in HIV primary care settings,
“it has the potential to improve access to ART and reduce morbidity and mortality among HIV-infected opioid-dependent patients who have traditionally been less likely to access and adhere to ART.” 76
Who Can Prescribe Buprenorphine?
In order to prescribe buprenorphine, you must be a licensed physician (MD or DO) and receive a waiver from SAMHSA's Center for Substance Abuse Treatment, and an accompanying ID number and Drug Enforcement Agency (DEA) registration number. (See "Qualify for a Waiver" below.)
How Many Patients Can I Treat?
Authorized physicians may treat up to 30 patients at any one time for the first year. After 1 year, physicians may submit a second notification, this one to treat up to 100 patients.
To access and email notifications, see the CSAT Buprenorphine Information Center On-Line Notification Form to Increase Patient Limit.
To access an online form to fax or mail in, see the CSAT Buprenorphine Information Center Pre-Filled Notification Form to Increase Patient Limit OR Download the PDF Notification Form and send to the contact information below:
Substance Abuse and Mental Health Services Administration
Division of Pharmacologic Therapies
Attention: Opioid Treatment Waiver Program
One Choke Cherry Road, Rm 2-1063
Rockville, MD 20857
Fax 240-276-1630
Phone 866-287-2728 (866-BUP-CSAT)77
(To learn more about increasing patient limits, visit the CSAT Buprenorphine Information Center, SAMHSA)
Conduct a Needs Assessment
Part of successful integration of opioid abuse treatment into your clinic is first identifying the level of abuse, including among those individuals potentially overlooked. The best way to do this is to implement screening programs to detect illicit drug use.
In cases where such screening isn't possible, providers may be able to utilize indirect estimates of illicit opioid use by using surrogate markers such as the number of patients with IDU as an HIV transmission category. Another surrogate marker is the number of clients receiving opioid prescriptions for chronic conditions.
National data, as well as clinical experience [from a SPNS Buprenorphine Initiative grantee] suggest that prescription opioid abuse may be proportional to the number of opioid prescriptions written.78,79
Are There Any HIV Drugs That Should Not Be Taken With Buprenorphine?
Buprenorphine, along with several HIV drugs, is metabolized by the liver. Clinicians need to be aware of drug-drug interactions. Select appropriate antiretroviral agents and monitor patients accordingly. To assist in guidance, see Table 2. Drug-Drug Interactions.
What Happens If Someone Needs Pain Medication With Buprenorphine?
Should patients need the use of a full agonist for pain relief management, buprenorphine should be discontinued until pain is able to be controlled without the use of opioid pain medications. Physicians should recognize, however, that use of opioid pain medications by patients with opioid addiction may further increase tolerance and physician dependency--counter to the goals of buprenorphine treatment. 53
Can People on Methadone Switch to Buprenorphine?
Yes, people can switch from methadone to buprenorphine, especially if they are working with an experienced physician. Since methadone is a long-acting opioid, people who want to switch should initially be treated with buprenorphine monotherapy (to avoid withdrawal symptoms from naloxone) before switching to buprenorphine and naloxone.
Before patients switch, they need to gradually taper their methadone dose (30 mg per day is recommended). 53 Physicians must contact the patient's Opioid Treatment Program (OTP) to ascertain the amount and time of the patient's last dose of methadone.
What Do I Need To Do To Get Started?
Qualify for a Waiver
Do you hold 1) a subspecialty board certification in addiction psychiatry from the American Board of Medical Specialties, OR 2) an addiction certification from the American Society of Addiction Medicine, OR 3) a subspecialty board certification in addiction from the American Osteopathic Association?57,80
If yes to any of the above, see "Submit a Waiver below. If no, see "Training below.
Training
Physicians must complete 8 hours of approved training from a public or private certifying board in treatment management of opioid-dependent patients. This may be done in person or online. This was the most common route pursued by SPNS grantees. The following websites list qualifying physician trainings:
- CSAT Buprenorphine Information Center Training Events Page
- American Academy of Addiction Psychiatry
- Buprenorphine Training Program Calendar, Opt-In
- BupPractice.com: Buprenorphine (Suboxone) Training and Practice Tools
Addiction may also be "medicalized like other chronic diseases and as such, principles of the chronic disease model that physicians may be using can also be applied."45 Several SPNS grantees also underscored this point. (To read about the Chronic Disease Model, visit the Improving Chronic Illness Care website.)
Submit a Waiver
Physicians must receive a waiver from the special registration requirements of the Controlled Substances Act in order to prescribe medication-assisted opioid treatment.80
Notify SAMHSA's Center for Substance Abuse Treatment of intent to dispense or prescribe opioid therapy. This step must be done prior to dispensing or prescribing treatment. Notification may be submitted online, by fax, or by standard mail.
Visit the CSAT Buprenorphine Information Center to submit a waiver form online. After filling out all required information, click on "Submit Waiver Notification."
Waiver forms (found at the address above) may also be downloaded and mailed or faxed to the following address:
Substance Abuse and Mental Health Services Administration
Division of Pharmacologic Therapies
Attention: Opioid Treatment Waiver Program
One Choke Cherry Road, Rm 2-1063
Rockville, MD 20857
Fax 240-276-1630
Phone 866-287-2728 (866-BUP-CSAT)77
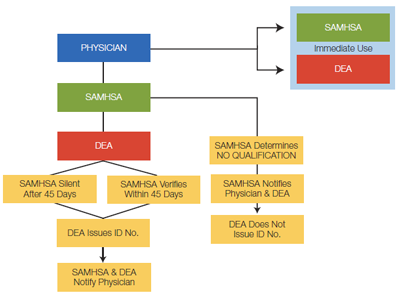
The office-based treatment notification review process is outlined in Figure 3 below. Physicians will be notified whether or not they've been granted opioid treatment prescribing privileges.
After the review process has taken place (typically, 45 days), approved physicians will receive an ID number and DEA registration number, which will need to be included on all prescriptions for opioid addiction therapy. (To learn more about waiver qualifications, visit the CSAT Buprenorphine Information Center.)
Will I Need To Offer Counseling Services?
Yes, physicians must be able to offer counseling services or have formal referral systems in place to link patients undergoing opioid treatment to counseling. (To view a grantee example of referral procedures, visit BEEHIVE Buprenorphine Program: Protocols.)
Counseling as a part of substance abuse treatment typically results in greater reductions in opioid and other substance use and greater adherence to buprenorphine and other medical treatment.81 Counseling can be offered onsite or though referral to offsite mental health services partners in the form of individualized counseling or weekly group counseling. Regardless of where it is administered or the format in which it is delivered, patients who attend counseling consistently achieve better treatment outcomes than those with little or no counseling.82,83
Figure 3: Office-Based Treatment Notification Review
Patients with the most success had the following characteristics:
- Access to therapy with a focus on comprehensive case management,
- A patient advocate with whom they felt they had good rapport and trust,
- Medical and psychiatric care and counseling that were closely linked, and
- Active engagement in their project participation.69
Confidentiality
Records for substance abuse treatment have stricter standards than traditional medical records.84,85 "This means either keeping separate records for the substance abuse treatment components of care or identifying records concerning substance abuse treatment in a way that they are released only under appropriate circumstances." 86 Use of electronic medical records, however, may assist in integrating HIV primary care records with opioid treatment records while still remaining in compliance with confidentiality regulations.85
Federal regulations require physicians to obtain signed patient consent before disclosing any identifiable patient information to a third party. To avoid any confidentiality issues, physicians are recommended to have a separately signed form for each patient.
According to SAMHSA,
It is particularly important to obtain patient consent when telephoning or faxing prescriptions to pharmacies, as this information constitutes disclosure of the patient's addiction treatment. When physicians directly transmit prescriptions to pharmacies, further redisclosure of patient-identifying information by the pharmacy is prohibited, unless signed patient consent is obtained by the pharmacy.87
This rule, however, does not apply if patients themselves deliver the buprenorphine prescription directly to the pharmacy.53 Suboxone package labeling also contains information on Federal confidentiality and regulations.
In addition, it's particularly important to educate phone room and front desk staff or to develop policies and procedures in collaboration with front desk and phone room staff. This staff can also be helpful in giving screening forms to patients when they come in. If everyone fills out forms, it reduces any stigma associated with them and patients don't feel like they're being singled out.
Ensure Appropriate Storage
Suboxone was required to be in a locked cabinet for storage when onsite at the SPNS grantee clinic sites. According to Federal regulations, buprenorphine of any kind must be stored in a securely locked, substantially constructed cabinet whereby patients would not have easy access and clinicians can accurately record the amount of medication received and dispensed. Any theft must be reported to the local DEA office in writing within one business day.88
Determine Available Clinic Space and Appointment Slots
SPNS grantees ensured they identified any structural changes that needed to be made to day-to-day operations of clinical services. This included assignment of clinic exam rooms and appointment slots in the clinic schedule. Patients need access to clinic space for the duration of their induction process as well as for routine check-ups.
Because medical interventions are rarely necessary during induction, regular office space could be used. Some grantees, however, found that offering induction in the same or similar area as future monitoring may be of assistance since patients are always returning to the same space.69
Secure Buy-in
Stakeholders were considered patients, medical providers, clinic staff, clinic administration, and pharmacy (inhouse or within the community). SPNS grantees found it was helpful to inform the community about their work. This allowed grantees to educate the community on buprenorphine and ensure this work was seen as a complement to--rather than a competition with--other available treatment alternatives, such as public and private methadone clinics, and residential detoxification and rehabilitation facilities, as well as substance-use treatment providers. Because of the cross-section of illegal opioid use and the criminal justice system, several SPNS grantees ensured outreach to their local jail services programs.69
Higher level stakeholders included staff, directors, or administrators of State AIDS Drug Assistance Programs (ADAPs) and State Medicaid Programs (to discuss buprenorphine and its potential inclusion on formularies), as well as any State and local offices of AIDS services, and narcotic enforcement officials.69
To continue support from personnel, SPNS grantee staff gave updates and case study presentations at monthly provider and clinic staff meetings. They also advertised the program by putting up flyers around the clinic and at affiliated partner sites. Clinic outreach workers also assisted with word-of-mouth advertising and education about the program in the community.
Patients who had successfully undergone buprenorphine induction were encouraged to speak to new patients about any fears they might have regarding the need to be in "withdrawal" at the time of induction. Hearing patient success stories helped alleviate fears.69 Patient brochures and informational sheets were also created for this purpose and can be accessed on the TARGET Center website:
The Communication Highway
SPNS grantees emphasized the importance of educating all staff about buprenorphine treatment. In particular, this assisted in cultural competency and improved communication and program success.
For example, some patients on opioid abuse treatment may be requesting opioid prescriptions (e.g., codeine, hydromorphone, morphine, oxycodone) from other health care providers that they see. As such, physicians integrating buprenorphine into their HIV clinics should be clear and communicative with others involved in patient care.
Determine Staffing
Several roles emerged as essential to successfully implementing opioid abuse treatment. These roles may overlap or be assigned to different individuals within your clinic.
- Clinical champion within your clinic to serve as a resource throughout the implementation process.
- Front desk and phone triage staff coached on clients presenting in opioid withdrawal or contacting the office to request opioid abuse treatment.
- Medical assistants and nursing staff prepared to work with patients in withdrawal.
- Coordinator to ensure things run smoothly; oversee referrals for housing, legal services, transportation, food pantry, clothing, individual counseling, and weekly buprenorphine support group counseling; and manage follow-up induction protocol.
- Office administrator responsible for correspondence, ordering supplies, and budget oversight.
- Substance abuse counselor with dedicated time for counseling clients who are seeking and receiving buprenorphine.
- Designated staff member to address benefits and insurance issues.
In many cases, these roles can be filled by existing staff who are already performing similar functions for other PLWHA within the clinic. Often, these responsibilities were part of the work assigned to what the SPNS Buprenorphine Initiative grantees came to call the “glue person.”
The “Glue Person”
Successful SPNS programs relied on a dedicated person who served as the face of the program within the clinic and the primary point of contact for issues related to buprenorphine. Providers—and patients—relied on this person deemed a “glue person,” although their background and training prior to the SPNS initiative varied from site to site. The glue person was not the primary clinician administering buprenorphine. Nevertheless, the glue person was imperative to the success of the program. Together, the physician and glue person created the Dyad Model—a two-person team taking responsibility for the program.69
Continuity, consistency, and stability are all essential components of addiction treatment, and the glue person helped provide that
The glue person was cited as a critical aspect, particularly at clinics where clinicians may do rounds and not be available every day. Creating such a team approach was also imperative to achieving clinic buy-in. Successful sites offered informational trainings to counter misperceptions and address hesitation to the implementation of buprenorphine into their clinic. (To assist in such trainings, see accompanying Buprenorphine Curriculum Guide from HRSA.)69
The glue person worked with the physician to develop and monitor individualized plans and track clinical outcomes. They provided patient education, conducted initial screenings and assessments for treatment eligibility, monitored and counseled patients during treatment (under the supervision of the clinician), and ran weekly patient support groups.69
As described by the University of California, San Francisco SPNS grantee, the glue person’s “presence in the clinic afforded [them] familiarity with most of the patients and providers, and this knowledge contributed greatly to the visibility of the program and to patient support.”
Although the background and education of the clinical care coordinator, or “glue person,” varied from site to site, all had some sort of substance abuse training and experience working within the fields of HIV and mental health. This was particularly helpful as co-occurring mental health issues are common among substance abusers. Clinic staff and patients alike were aware of the glue person’s role, and inclusion of that role helped support the success of these programs.
Identify a Mentor
SPNS grantees underscored the importance of having a physician mentor, particularly during the planning and early implementation steps of integrating opioid abuse treatment into their clinics. Specifically, grantees accessed the SAMHSA-funded Providers' Clinical Support System for Medication Assisted Treatment (PCSS), a free, nationwide program linking physicians interested in integrating buprenorphine into their practices with mentors for support. (See also The Clinical Education Initiative out of New York State's Department of Health). To chat with other DATA waiver-approved physicians, join the free SAMHSA buprenorphine clinical discussion Web Board.
Professional societies and HRSA's AIDS Education and Training Centers also offer HIV-related support.
Pharmacy Care
At one clinic, Suboxone sublingual tablets were dispensed from the HIV clinic directly. Then, as the patient progressed and achieved a stable buprenorphine dose, prescriptions were transferred to a community pharmacy.69 Several grantees found it helpful to tour a pharmacy to observe buprenorphine storage and dispensing practices. Grantees noted that onsite availability of a pharmacy and laboratory facilitated implementation and execution but were not prerequisites for success.
Table 2: Drug–Drug Interactions
| Buprenorphine: Effect on ARV Level | ARV: Effect on Buprenorphine Level | |
| Nucleoside Reverse Transcriptase Inhibitors | ||
| Abacavir (Ziagen; Trizivir and Epzicom also contain abacavir) | Not studied; monitoring recommended due to potential interaction.1 | Not studied; monitoring recommended due to potential interaction.1 |
| Didanosine (ddI; Videx tablet or EC) | Buprenorphine has no effect on ddI.2 | ddI has no effect on buprenorphine.2 |
| Emtricitabine (FTC; Truvada, Atripla, and Complera also contain FTC) | Buprenorphine has no effect on emtricitabine.1 | Emtricitabine has no effect on buprenorphine.1 |
| Lamivudine (3TC, Epivir; Combivir, Epzicom, and Trizivir also contain lamivudine) | Buprenorphine has no effect on lamivudine.2 | Lamivudine has no effect on buprenorphine.2 |
| Stavudine (d4T; Zerit) | Buprenorphine has no effect on stavudine.1 | Stavudine has no effect on buprenorphine.1 |
| Tenofovir (Viread; Truvada, Atripla, and Complera also contain tenofovir) | Buprenorphine has no effect on tenofovir.2 | Tenofovir has no effect on buprenorphine.2 |
| Zidovudine (AZT, Retrovir; Combivir, and Trizivir also contain zidovudine) | Buprenorphine does not cause significant changes in zidovudine levels.3 | Zidovudine does not cause significant changes in buprenorphine levels.3 |
| Non-nucleoside Reverse Transcriptase Inhibitors | ||
| Delavirdine (Rescriptor) | Buprenorphine does not cause significant changes in delavirdine levels.4 | Delavirdine increases buprenorphine levels but does not cause side effects/symptoms other than drowsiness.4 |
| Edurant (Rilpivirine; Complera also contains rilpivirine) | No data | No data |
| Efavirenz (Sustiva; Atripla contains Sustiva) | Buprenorphine does not cause significant changes in efavirenz levels.4 | Efavirenz lowers buprenorphine in bloodstream, but without causing withdrawal symptoms; although buprenorphine dose adjustment is unlikely to be necessary, monitoring for withdrawal symptoms is recommended.4 |
| Etravirine (Intelence) | Buprenorphine does not cause significant changes in etravirine levels.5 | Etravirine lowers buprenorphine levels; although buprenorphine dose adjustment is unlikely to be necessary, monitoring for withdrawal symptoms is recommended.5 |
| Nevirapine (Viramune) | Buprenorphine does not significantly change nevirapine levels.6 | Although nevirapine significantly lowers buprenorphine levels, buprenorphine dose adjustment is unlikely to be necessary.6 |
| Protease Inhibitors | ||
| Atazanavir (Reyataz) Atazanavir/r | Buprenorphine does not significantly change atazanavir or atazanavir/r levels.7 | Atazanavir and atazanavir/r increase buprenorphine levels; buprenorphine dose adjustment may be needed if opioid toxicity/side effects develop.7 |
| Darunavir/r (Prezista) | Buprenorphine does not cause significant changes in darunavir/r levels.8 | Darunavir/r increases buprenorphine levels; dose adjustment is not necessary, although clinical monitoring is recommended.8 |
| Fosamprenavir/r (Lexiva) | Buprenorphine does not cause significant changes in fosamprenavir/r levels.9 | Fosamprenavir/r does not cause significant changes in buprenorphine levels; dose adjustments unlikely to be needed.9 |
| Indinavir/r (Crixivan) | No data | No data |
| Lopinavir/r (Kaletra) | Buprenorphine does not significantly change lopinavir/r levels.10 | Lopinavir/r increases clearance of buprenorphine’s main metabolite (called norbuprenorphine, or norBUP), but this does not cause side effects and buprenorphine dose adjustment is not necessary.11 |
| Nelfinavir | Buprenorphine does not significantly change nelfinavir levels.10 | Nelfinavir does not significantly change buprenorphine levels.10 |
| Ritonavir (Norvir) | Buprenorphine does not significantly change ritonavir levels.10 | Ritonavir increases buprenorphine levels, but no dose adjustment is needed.10 |
| Saquinavir/r | No data | No data |
| Tipranavir/r (Aptivus) | Buprenorphine lowers tipranavir/r in the bloodstream by 19 to 25 percent; use with caution. Therapeutic drug monitoring may be needed.12 | Although tipranavir/r lowers the level of norBUP, this does not cause side effects, and buprenorphine dose adjustment is not necessary.12 |
| Integrase Inhibitors | ||
| Raltegravir (Isentress) | Buprenorphine does not significantly change raltegravir levels.13 | Raltegravir does not significantly change buprenorphine levels; buprenorphine dose adjustment is not necessary13 |
| Entry/Fusion Inhibitor | ||
| T-20 (Enfuvirtide; Fuzeon) | No data | No data |
| Maraviroc (Selzentry) | No data | No data |
